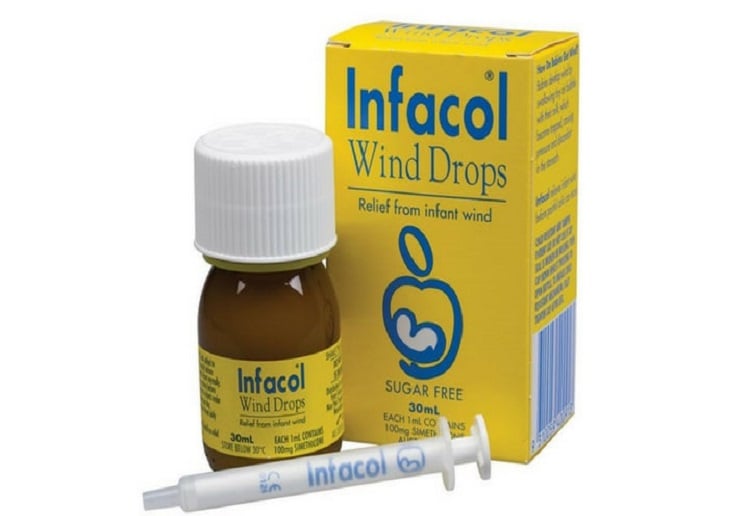
TGA have announced a recall for three batches of Infacol Wind Drops oral Liquid Bottle.
The recall only affects retailers, not consumers, there is no risk of harm, according to Kidspot.
Batch no:
CM832 Expiry: November 2019
CP277 Expiry: April 2020
CP658Expiry: June 2020
Recall Issue: Nice Pak Products has been made aware by the manufacturer that batch CM832 is Out of Specification for Description (contents) and Assay of polydimethylsiloxane. The action has also been applied to the two batches manufactured following CM832. This recall does not affect any other batches of Infacol Wind Drops 30mL.
Please note this is a Trade level only recall, of withdrawal of stock from stores. Based on product quality and not product safety issues. There is no consumer action that needs to be taken. The use of the product is unlikely to cause any patient health risk.

Share your comments below
We may get commissions for purchases made using links in this post. Learn more.






















-

-
-
-
mom101628 said
- 09 Sep 2018
-
-
-
-
mom81879 said
- 19 Jun 2018
-

-
-
-
mom160421 said
- 04 Jun 2018
-
-
-
-
Cookfromscratchmum said
- 04 Jun 2018
-

-
-
-
Ellen said
- 04 Jun 2018

Post a comment4:36 pm
9:44 am
1:37 pm
1:09 pm
11:30 am
To post a review/comment please join us or login so we can allocate your points.