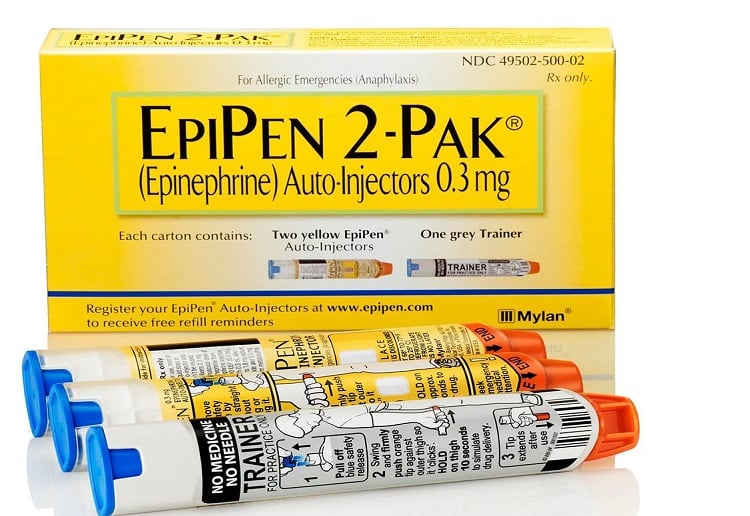
A RECALL has been issued for over 80,000 EpiPens.
Four batches of EpiPen 300 microgram adrenaline are being recalled due to a defective part affecting the auto-injector.
The Alphapharm-branded EpiPens require more force to deliver a shot of adrenaline, or can fail to activate the auto-injector altogether.
“EpiPens are only used in emergency situations,” says the recall notice.
A person suffering from a severe allergic reaction who was issued with one of the defective EpiPens could experience “a worsening of the potentially life-threatening symptoms”.
There have been two confirmed reports of auto-injectors failing to activate correctly. However the TGA says the proportion of the defective EpiPens used remains unknown.
Four batch numbers contain the defective part, all of which expire in April 2017. These include batches 5FA665, 5FA6651, 5FA6652 and 5FA6653.
The batch numbers and expiry dates of the affected EpiPen’s can be checked on either the label of the EpiPen or on the end of the carton.
The TGA is urging anyone with an affected EpiPen to return it to a pharmacist, either for a replacement or a refund.
DO you have one of the affected batches? How did this news make you feel?
Share your comments below.
We may get commissions for purchases made using links in this post. Learn more.



















-

-
-
-
mom94125 said
- 14 Oct 2017
Reply
-
-
-
-
mom70876 said
- 25 Apr 2017
Reply
-
-
-
-
june11 said
- 01 Apr 2017
Reply
-

-
-
-
tessie said
- 24 Mar 2017
Reply
-

-
-
-
Ellen said
- 24 Mar 2017

Reply
-

-
-
-
sars_angelchik said
- 23 Mar 2017
Reply
-
-
-
-
ella12 said
- 22 Mar 2017
Reply
-

-
-
-
rovermum said
- 22 Mar 2017
Reply
-
-
-
-
mom112217 said
- 22 Mar 2017
Reply
-

-
-
-
cherz said
- 22 Mar 2017
Reply
Post a comment8:30 pm
1:12 pm
8:26 pm
12:13 pm
3:04 am
10:15 am
2:35 pm
2:07 pm
2:00 pm
12:25 pm